FAQ
If you don’t find the answers you need below, please chat with us online, or contact us at 800-692-4380 or email us at info@fisherwallace.com. Live patient support is available seven days a week from morning to evening.
How to use
What is the recommended treatment protocol?
Customer should use the product twice a day for 20 minutes on Level 2, once after waking up for the day and again before bed. It is important to create a consistent routine of daily usage.
There are no withdrawal effects associated with decreasing or stopping use of the product.
What activities may I do while using the product?
Resting quietly is best during a treatment session, but it’s fine to do other activities such as reading, watching TV, using the computer, or talking on the phone.
How soon will I experience benefit?
Most customers experience benefit in the first 1-4 weeks of daily treatments, but some can see results even faster. The product should be used daily for at least 2 weeks before making a decision to keep it or return it.
Is there anyone who should NOT use the product?
The product should not be used on or near areas of the body, including the head, that contain implanted devices, such as stimulators, stents, or active or inactive implants such as deep brain stimulators and vagus nerve stimulators").
- Do not use this product around the Carotid sinus.
- If you have known or suspected heart disease, you should not use the product.
- The product should not be used by persons who have known or suspected trigeminal neuralgia.
- If you are pregnant, you should not use the product.
- If you have reacted poorly to the idea of electrical stimulation of any kind, you should not use this product.
Additional precautions:
- Discontinue use if your skin becomes irritated around either electrode site.
How it works
How can I tell if the product is working?
If the green indicator light AND at least one yellow light are illuminated, the product is delivering stimulation. If there is no yellow light illuminated, the sponges may not be sufficiently moist, or the batteries may need to be replaced.
What does the product feel like?
Many customers do not feel the product at all, while some may feel a mild tingling at the sponge contact sites.
How does the product help me feel better?
The product uses a technology called Transcranial Alternating Current Stimulation (tACS), which modulates brain function and cognitive processes by entraining neural oscillations and inducing long-term synaptic plasticity.
Are there side effects?
Less than 1% of patients report a mild, temporary headache or dizziness when using the product.
Although not a side effect, improper use of the product may result in minor skin irritation beneath the electrode sites - this can occur if the sponges are not thoroughly wet before use or if the scalp and/or hair are not clean. Please watch the instructional video and read the instruction manual thoroughly before using the product.
The product has been on the market for decades without any reports of long-term negative effects. Many customers have used the device for years.
How to assemble
How do I set up the product?
Please view our instructional video for an overview of the process. The product is very simple to use and inexpensive and easy to maintain.
How do I know if I assembled the device correctly?
If the green indicator light AND at least one yellow light are illuminated, the product is delivering stimulation. If there is no yellow light illuminated, make sure that:
- The sponges are thoroughly wet.
- The headband is wrapped tightly around your head.
- You have pushed your hair out of the way of the electrodes.
- The batteries are fresh/fully charged.
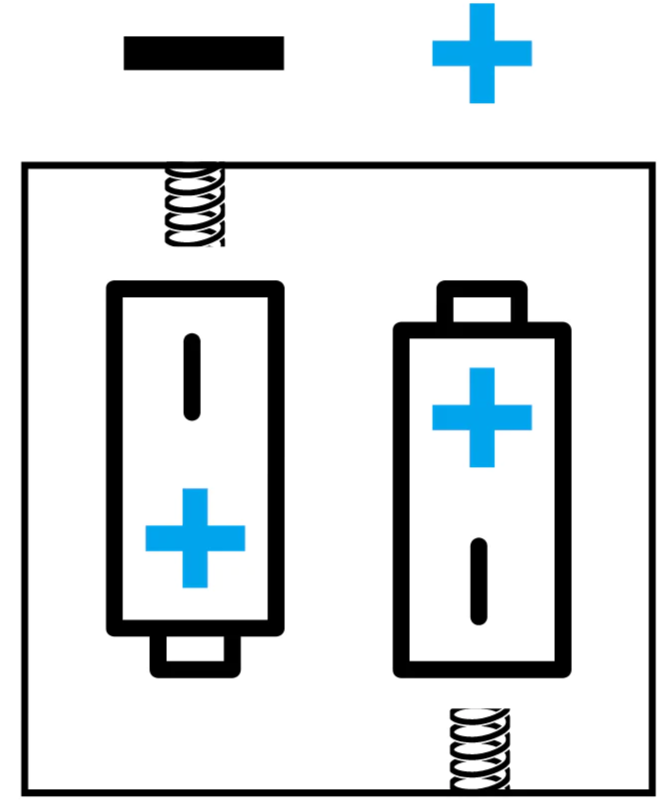
NOTE: Battery placement
Batteries must be placed into the battery compartment with correct orientation, as shown in the diagram, for the product to perform.