Clinical Research
Our patent-pending neuromodulation technology delivers low-dose, pulsed, alternating current stimulation (tACS) which, as a type of non-invasive brain stimulation, has been shown to induce neural plasticity and entrain neural oscillations.
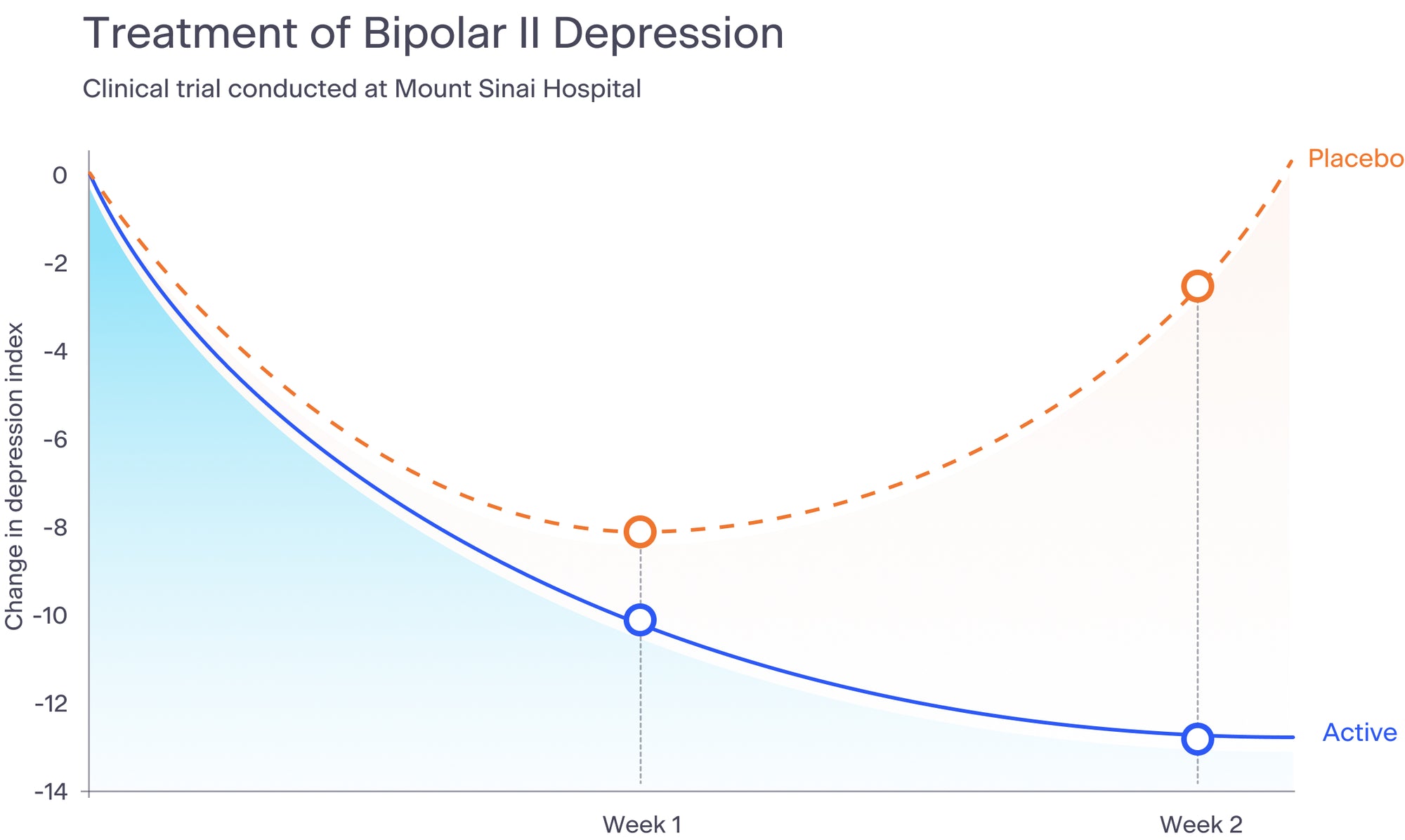
We have conducted multiple clinical trials, including a bipolar II depression study at Mount Sinai Beth Israel Hospital, a Parkinson's study at the University of Maryland, three pilot studies during the height of the pandemic, a pivotal depression trial in 2022 that will be used to obtain a CE Mark in Europe for the acute treatment of depression in women, and a real world evidence anxiety study with the Seattle Police Department. We are preparing to launch a new depression study to obtain FDA approval for the treatment of depression.
The results of our most recent depression study are being prepared for scientific journal publication. Below is a graph that summarizes the results of our bipolar II depression study, published in the Journal of Nervous and Mental Disease in 2015.